On October 4, 2022 at Petchachompoo Room, The Emerald Hotel Assoc. Prof. Siree Chaiseri, Ph.D., presided over the opening of the seminar: “Presentation of research results and listening to opinions regarding the “Assessment of policy on organizational structure and management to upgrade Thailand’s National Quality Infrastructure (NQI)”. The project was conducted under the support of Thailand Development Research Institute (TDRI) with an aim to propose a guideline for NQI improvement and a strategic plan for driving the NQI system in Thailand. The main objective was to analyze challenges and barriers within the NQI system, and to review roles and functions as well as authority of relevant organizations involved in NQI management for the country. NQI best practices from other countries were reviewed in order to make policy recommendations on the system management structure, to improve inter- agency cooperation, and to obtain a draft strategy for driving improvement in the NQI system in Thailand.



At present, research funding for advanced technology development presents inherent limitations in terms of complying with various standards. This creates a barrier to bringing research off the shelf and towards commercialization, making it more difficult to realize the full benefits of the research work. Therefore there is an overwhelming consensus among government agencies and related organizations from all sectors that the NQI system needs to be comprehensive, centralized, interconnected and well-developed for complete integration of the entire country. Therefore, TDRI was appointed to study the organizational structure and management to upgrade Thailand’s national qualitative infrastructure (NQI) and to draft a strategic plan to drive Thailand’s NQI system, as well as promote collaboration between the public and private sectors, which play a very important role to ensure the country’s NQI is developed to meet internationally accepted standards

Thailand’s National Quality Infrastructure (NQI) is a system comprised of public and private organizations that share the same policies, laws, regulatory frameworks, and operational guidelines. It can be divided into 5 components: 1. Metrology 2. Standardization 3. Accreditation4. Conformity Assessment, and 5. Market Surveillance.
The following is an example of a guideline for production and service provision in accordance with the 5 elements of NQI.
- Manufacturers must produce products that meet the standards specified by the standardization unit, such as the ISO standards for industrial products, or the Codex standards for agricultural and food products.
- The certification body will inspect and certify standardized products and services to build credibility and earn consumers’ confidence.
- An independent auditing authority shall assess the certification agency to reinforce confidence, in the accreditation of the service as mentioned in clause 2.
- Metrology will stipulate accurate measurements to be adhered to by the inspection bodies in accordance with national measurement standards.
- Products and services entering the market will be monitored by market regulators before and after entering the market. If it is found that the products or services do not meet required standards there is a protocol to extract the product or service out from the market.
Ultimately, for the 5 elements mentioned above to be applied effectively, there must be a governing agency to set clear and integrated set of policies and direction for the development of the NQI system throughout the country so that audit standards are transparent and credible internationally, giving Thai products and services the potential to compete in the global market.
A cursory study of foreign NQIs by the TDRI presents these interesting findings:
Germany has a clear autonomous NQI regulatory agency, where the government is responsible for supervising metrology, certification and market regulation, while the private sector is in charge of stipulating standard requirements. Comparatively, in China and Taiwan, the NQI system is operated entirely by the state, except for the inspection and certification process, which is conducted by the private sector with zero intervention by the state.
In the United States, the private sector is the main party responsible for setting standards, which represents true market requirements, thus promoting inclusiveness and balance of trade. Incidentally, the vast majority of foreign NQIs follow UNIDO’s guidelines.

UNIDO, or the United Nation Industrial Development Organization, which is a special type of organization under the United Nations General Assembly, with the duty to promote and accelerate the industrial development of developing countries, especially in the manufacturing sector. Efforts are being made to help developing countries take a more active role in the formulation and implementation of the national NQI standards, thereby increasing their
readiness to enter the global market
White Paper on National Quality Infrastructure Reform
The process of drafting the National Quality Infrastructure Act required setting up a National Quality Infrastructure Policy Committee, which has the authority to formulate the NQI policy and master plan for the entire country. The Statute on NQI was prepared to be used as a framework and guideline for formulation of relevant NQI policies, strategies and operational plans with a proposal to organize NQI units into 3 groups as follows: The main national organization group (Metrology, Product Standards Office, and accreditation organizations), inspection and certification group, and the service recipients group. The proposal shall be prepared for submission to the government cabinet for approval, which will help close the gap in the country’s NQI system, resulting in overall national integration, reducing the overlap of roles and responsibilities of various agencies so that the national NQI system can be developed in line with the development of technology and innovation, while responding to the needs of the international market.
Guidelines for the development of an NQI system for key industries in the country

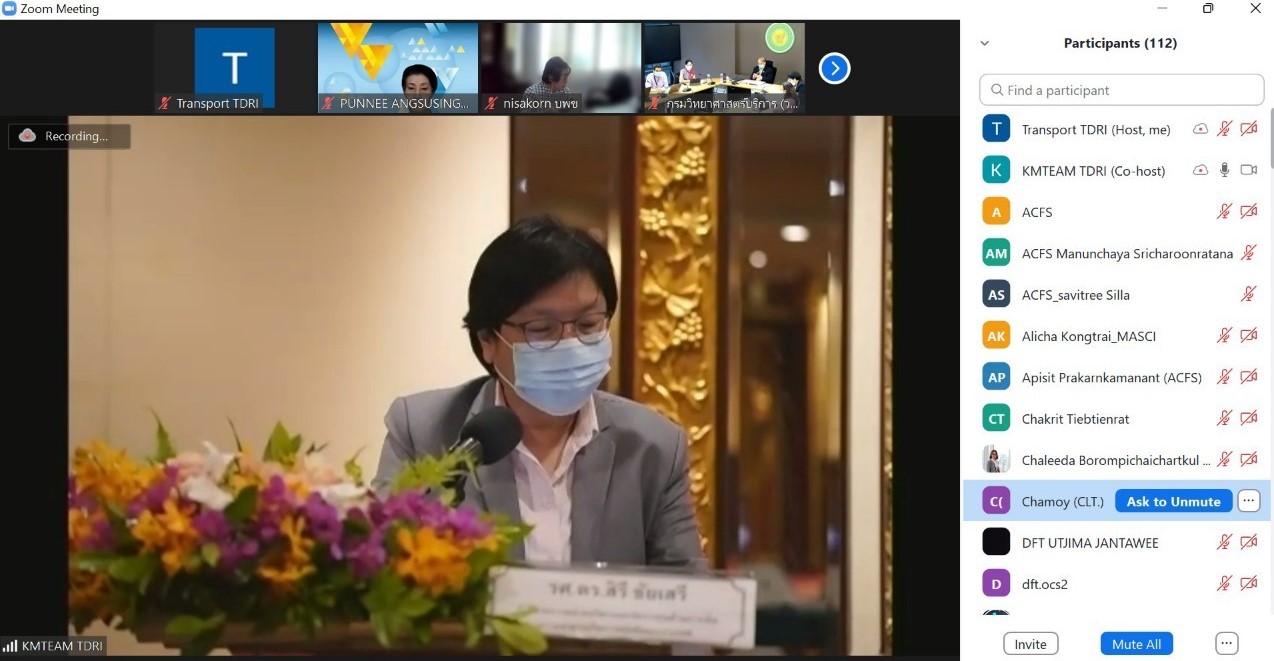
The main problem for the Thai rice industry is that Thai rice varieties do not meet the needs of consumers. Because Thailand exports cheaper, lower-grade hard rice, while the foreign market demands soft rice varieties. Other problems include issues with production quality, high costs, and low level of support for research on Thai rice. Improving the Thai NQI system to solve problems in the Thai rice industry includes starting from the policy level for rice production to meet the market needs; maintaining centralized infrastructure-related data to support the producers, to encourage the private sector to compete on quality standards; developing certification standards such as adding a certification body, or hiring additional support from the private sector while developing more auditing and certification capabilities; developing research and development on rice production and setting guidelines for systematic market surveillance.

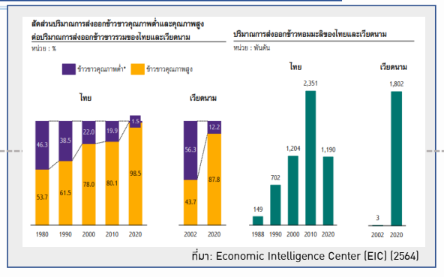
A comparative study of the global market for retail herbal products in 2021, the total market was worth 1.76 trillion baht and the herbal product market in Thailand was worth 45.6 billion baht (about 2.6% compared to the world market). However, Thailand imported a total of 12.6 billion baht worth of herbal extracts and supplements in year 2020, even though Thailand possesses some of the most fertile soil and a vast variety of herbs. The main problem of the herbal industry in Thailand stems from the lack of knowledge and understanding of cultivation to meet quality
standards and requirements, as well as having a scarce number of certified laboratories. As a result, the amount of high quality herbal extracts is insufficient. In addition, Thailand lacks research and development or clinical study data to be used to support health claims from herbs. Therefore, the proposal for the development of Thai NQI focuses on promoting the production capability starting with the upstream producers, specifically educating farmers on the required practices to meet necessary standards; increasing research and development to help herbal
products meet international standards; increasing the number of inspection and certification agencies to support the GAP or Organics standard certification process; increasing the number of OECD-GLP standard laboratories, including establishing clear guidelines for random inspection of herbal products in the market.
Photo 3: Development of NQI to increase potential in the automotive and auto parts industry.

Thailand exports automobiles, automotive parts and tires, averaging in value of 1.3 trillion baht per year (2017 – 2021) Source: Office of Industrial Economics.
But when comparing to developed countries, Thailand has a lower proportion of standards that comply with UN UNECE Regulations. As a result, the exported vehicles and parts to foreign countries must be retested at the destination country.
In addition, Thailand also has safety standards for vehicles and auto parts that are not yet fully compliant with the standard recommendations provided by the International Organization of Automobile Manufacturers. Moreover, there still is insufficient number of domestic inspection and certification agencies. Therefore, the proposal for NQI development for the automotive industry includes the development of standards for automotive and automotive parts to be fully complaint as specified in the UN Regulation. This includes raising the safety standards of vehicles and automotive parts throughout the country, stipulating a comprehensive set of safety technical regulations, including accelerating the development of various testing and certification bodies to support standardized testing in the automotive industry.
Photo 4: Development of NQI to increase potential in the medical device industry.
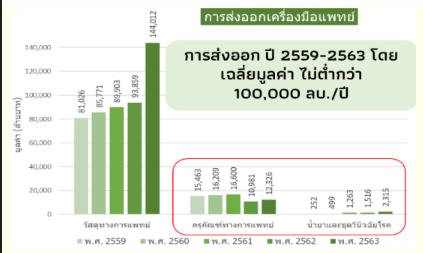
In the medical device industry, most Thai business operators are SMEs that produce medical consumables. However, Thailand is still unable to produce all types of important medical equipment, because the domestic market does not offer sufficient demand and scale to justify investing in manufacturing plants. Additionally, most hospitals still prefer to use imported products from abroad.
In terms of the country’s NQI system, there is still a lack of a regulatory agency for the production of medical devices. GMP certification for manufacturing plants is still not sufficient to provide certification for all factories. Also, entrepreneurs do not have sufficient knowledge about required standards testing for exports. As well, there is a large insufficiency in terms of infrastructure for laboratory testing and calibration to support the needs of operators
Proposal for the development of NQI for standardization of the medical device industry includes: standard specification, especially for targeted products, creation of domestic markets through government procurement mechanisms, and promoting standards-related knowledge database provided by government agencies. As for the overall policy guideline, there should be a primary agency to drive and push for medical device innovation, including the preparation of a database of usage requirements for medical devices, as well as an overview of the market trends to support business operators.
For the long term development of inspection and certification, a comprehensive medical device testing center should be established, as well as an adequate product certification infrastructure.







