Program Management Unit for Competitiveness (PMUC)
Nowadays, people all over the world pay great attention to the issue of health care for themselves and their family. This is evident in the growth of the medical and health businesses and industry. And Thailand is one of the countries considered to have high potential in the medical and health industries. This is due to the government’s policy that aims to make Thailand a center for health and medicine (Medical Hub) at the international level. But the question is are we ready to step up to that level today?
Most Thai people think that Thailand is becoming a center for health and medicine, but in fact Thailand rather stands out as a Medical Service Hub. In the past, Thailand gave little attention to medical industry, and as a result, the country has to import hundreds of billions of baht in pharmaceutical products and medical devices each year.

Prof. Sansanee Chairote, Ph.D., chairperson of the Health and Medicine Program Subcommittee, Program Management Unit for Competitiveness (PMUC), Office of National Higher Education, Science, Research and Innovation Policy Council (NXPO), revealed that the medical industry in Thailand is an industry that is not growing or developing as much as it should because it relies heavily on importation of drugs and medical equipment. Most of the drugs that we produce ourselves are those that have expired patents, while there are very few Active Pharmaceutical Ingredients (APIs) that we can manufacture ourselves.
“With the coronavirus disease 2019 (COVID-19) epidemic, everyone realizes that the global value chain congestion in the form of delayed shipping, or reduced production, had large impacts on medical and public health care sectors. Therefore, all countries around the world need to pay more attention and turn to rely on themselves, especially by promotion of medical innovations so that the country can move forward in spite of the pandemic that cuts off means of communication and transportation. PMUC, as an organization responsible for the development of Thailand’s competitiveness, therefore plays an important role in supporting and upgrading the Thai health and medical industry to be more competitive in the global market.”
Prof. Dr. Sansanee continued that the method of providing investment capital by PMUC will focus on the form of “Matching Fund” which is a cooperation or joint investment with the fund recipients. Normally, the private sector will be involved in research only when such research is at the Technology Readiness Level (TRL) of TRL 7-8-9, which are the final stages before entering the commercial market. However, the support of PMUC’s investment will allow the private sector to participate from the TRL levels 4-5-6, which is the “Translation Research” phase and the “Scaling up” period or the period during which the focus is to increase production capacity to meet commercial levels. It’s important to get the private sector involved and investing in technology from the start of the process. In the past, professors or researchers in the public sector often performed academic research. Due to the changing era, however, there should be more interaction and collaboration with the private sector, and attention should be given to the needs of the business sector to obtain investment information as well as marketing requirements, to be able to transfer innovations to commercial processes.
However, in the Health and Medicine Program, PMUC does not focus solely on providing research funding, but it also places importance on building the capacity of research personnel in the Translation Research aspect, as this group of personnel should possess expertise in bioengineering with multi-tasking capability to meet the level of technological readiness at TRL 4-5-6. Furthermore, PMUC also organizes training activities to prepare researchers in terms of product quality control according to various standards, such as the standards of the Food and Drug Administration (FDA) or ISO standards. Also part of the program’s initiatives is the creation of new technology platforms and providing funding to agencies who possess high potential in medical research. It is an important fundamental factor that PMUC has not neglected. PMUC offers support for building standardized prototype factories such as Advanced Therapeutic Medical Product (ATMP) manufacturing laboratories that meet Good Manufacturing Practice (GMP) standards. Such effort is essential in enabling researchers to continue their research to invent new innovative medical treatments.
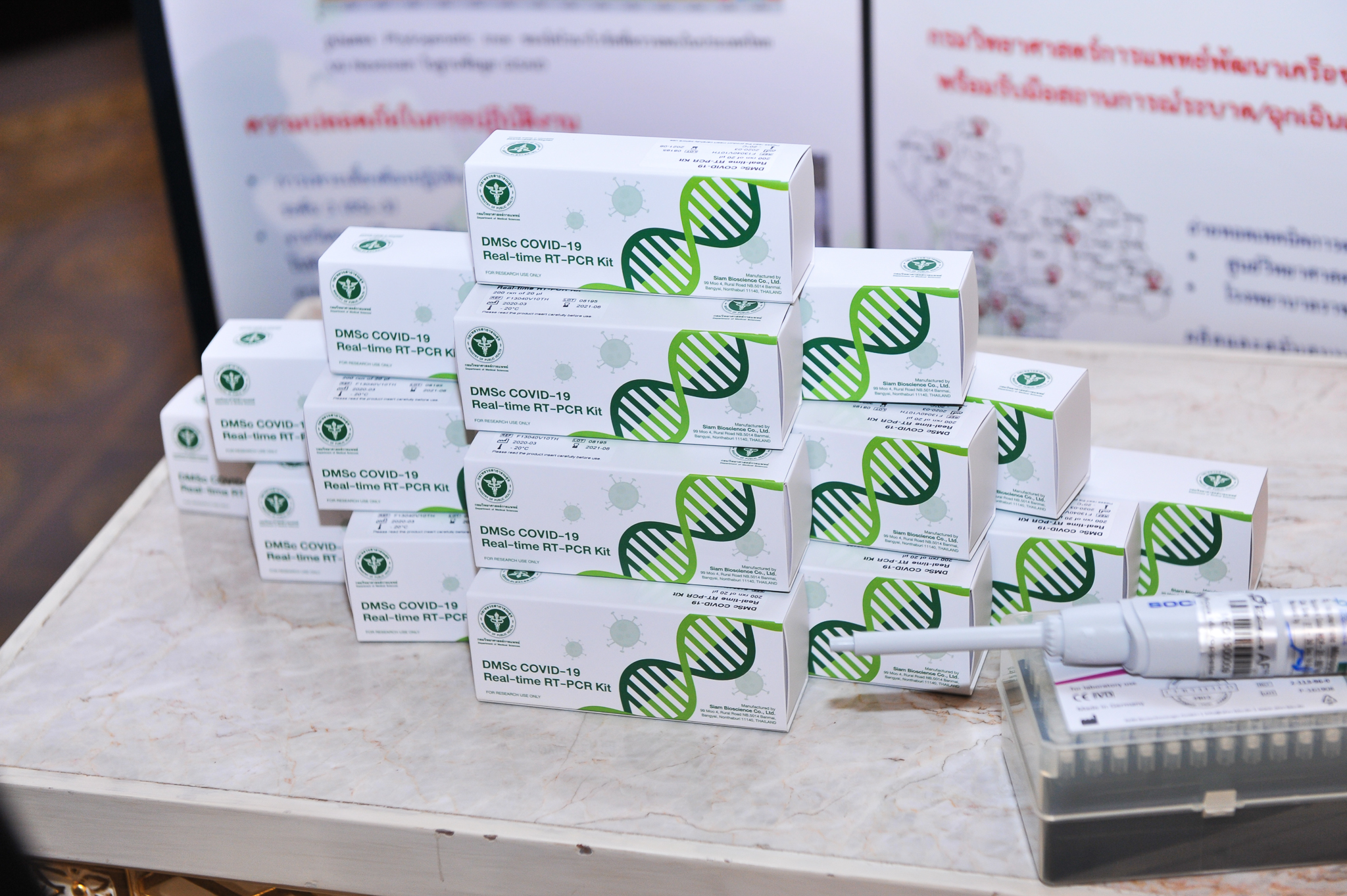
Prof. Dr. Sansanee said that during this time that Thai people are facing the COVID-19 crisis since 2020 PMUC is trying to find a solution to the crisis by supporting research and development of the COVID-19 diagnostic test kit using RT-PCR technique, which is a collaborative effort among researchers from the Department of Medical Sciences, Ministry of Public Health, together with Siam Bioscience Co., Ltd. by transferring relevant technology to the company legally, and obtaining FDA standard approval for them to produce SAR-CoV-2 test kits using real-time RT-PCR method, enabling Thailand to have sufficiently standardized and inexpensive COVID test kits for domestic use and can be distributed to ASEAN countries according to government policy.


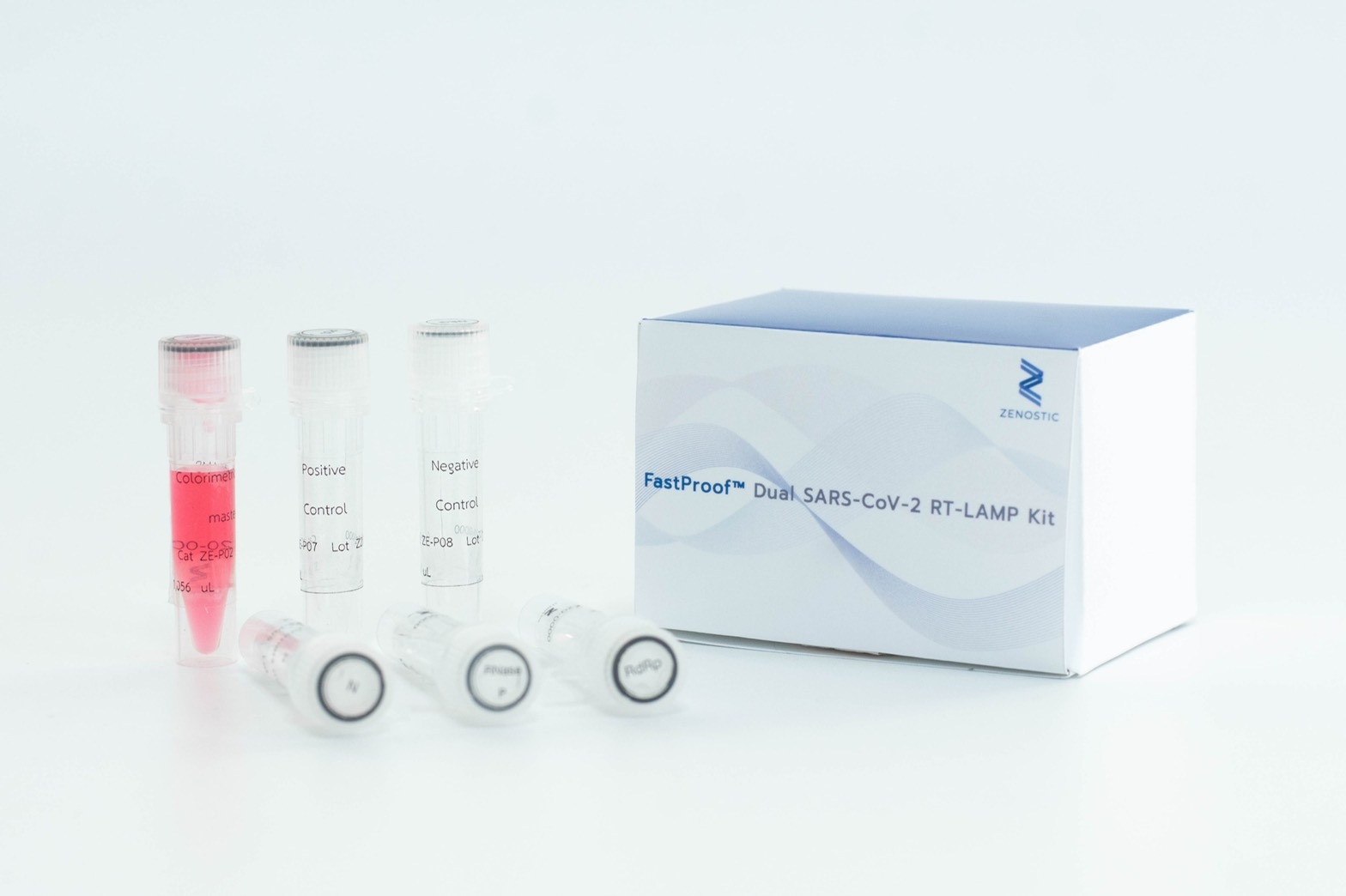
On the subject of support for the “Covid-19 test kit using the lamp technique”, PMUC has provided capital support to startup companies, allowing them to produce test kits that have been evaluated by the Department of Medical Sciences, Ministry of Health, which is a good thing because this method is faster and cheaper than the RT-PCR technique. Although providing lower levels of sensitivity and accuracy, but it provides standard level initial screening in the event of a widespread outbreak. In this particular case, PMUC has worked with the Food and Drug Administration (FDA) to set a new standard for testing using lamp technique according to academic principles and international quality standards, giving Thailand the opportunity to utilize this type of test kits.

As for the questions about overall objectives and important achievements expected in the area of health and medicine in the next 5-10 years, PMUC aims for: 1). 3 new drugs, which are biologic drugs that Thailand can produce on its own and register a patent protection for. 2). at least five ready test kits and laboratories that can be sold commercially within the country and abroad. 3). a laboratory that meets international standards, which offers capabilities for gene therapy, which is a type of treatment called “Precision Medicine”, which is a new hope in the global medical community. 4). successful extraction of plant substances that meet international standards, which can be used as a dietary supplement or ingredient in the treatment of diseases 5). establishment of a Clinical Research Organization (CRO) company, which Thailand does not yet have a company arising from such cooperative effort, and 6).the emergence of new high technologies (Deep Technology) in Thailand and the use of digital technology to collect medical and public health information for use in the diagnosis, treatment and control of diseases, all of which will generate income for the country from the health and medical industries. as well as enabling Thais to have greater access to medicines and public health systems.
“We often say that Thailand is stuck in the middle income trap because we invest in technology that creates low value. But medical health products are things that create very high value. If we can produce to meet the needs of the country, it can help to reduce imports and exports, and it will allow us to generate enormous income for the country. Increasing market competitiveness in exporting various health and medical products, combined with the strengths of Thai people in the service sector, will definitely help Thailand get out of the middle income trap. Moreover, it could help contribute to the goal of becoming an international health and medical hub. Thailand can definitely move towards achieving that goal, if only Thai people will be more assertive and focus on “quality innovations”, because innovation is not just a new invention or process, but also requires bringing these inventions and processes to create value and, in turn, increasing the country’s competitiveness in industrial and economic aspects, all which can contribute to overall social development for the country, Prof. Dr. Sansanee said in closing.







